
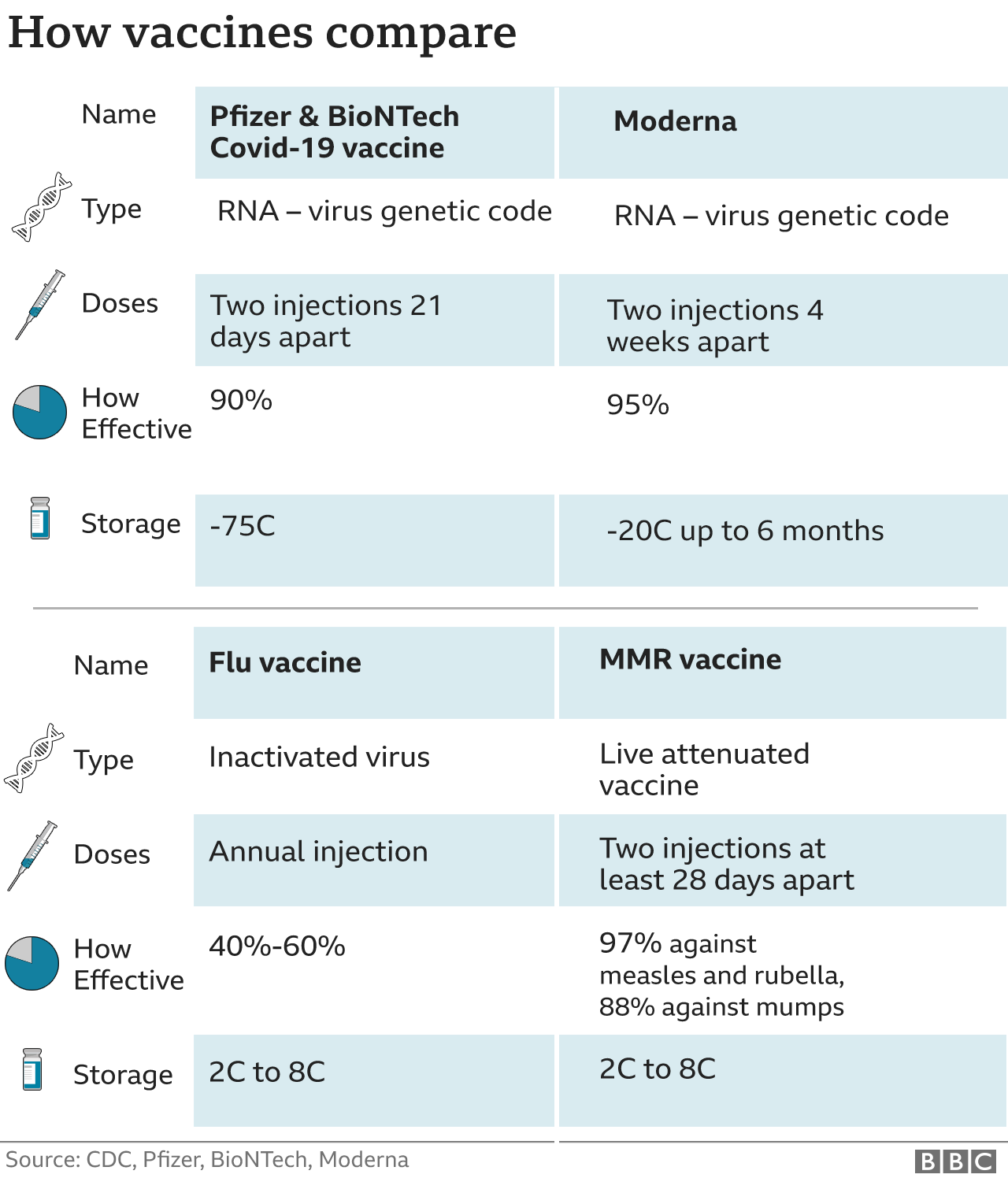
The Omicron variant emerged in late November 2021.Ĭritical illness was considered hospitalization with high-flow oxygen, mechanical ventilation, extracorporeal membrane oxygenation (ECMO), or kidney dialysis within 28 days of COVID-19 diagnosis.Īmong the 3,203,985 adolescents, 57.4% were 12 to 15 years old, 27.6% were 16 or 17, and 15.0% were 18. In a related study published yesterday in JAMA Network Open, researchers compared the rates of COVID-19 infection and critical infection by age, region, vaccination status, and number of Pfizer COVID-19 vaccine doses in all adolescents aged 12 to 18 years in South Korea from Jul 19, 2021, to Jan 22, 2022. There were no reports of myocarditis (inflammation of the heart muscle) or death. There were three reports of serious events: new-onset type 1 diabetes 10 days after vaccine receipt, facial swelling 3 days after receipt, and generalized pain, fatigue, and malaise after 5 days, the latter of which required hospitalization.
Other frequently reported events included fever (7.8%), limb pain (6.6%), and fatigue (4.8%). The most common nonserious events (71%) were related to vaccine preparation or administration errors (eg, incorrect dose, inappropriate patient age), and 15.3% of the 413 reports also included an adverse health event. Nearly all (99.5%) of reports were considered not serious. Over the study period, VAERS received 581 reports of one or more adverse effects after third-dose COVID-19 vaccination. About 1.0% of parents said they sought medical attention for their child after the third dose (vs 0.9% after dose 2), most often at a clinic (0.5%) or through telemedicine (0.3%). While most adverse effects reported by those who experienced pain, fatigue, headache, or muscle pain were mild, more moderate and severe symptoms were reported after the third dose than after the second.ĭuring the week after the third dose, 6.9% of children didn't attend school because of adverse effects (vs 10.0% after dose 2), and 12.1% couldn't participate in daily activities (vs 7.5% after dose 2). Injection-site and systemic reactions had been documented in similar proportions after the second dose (68.0% and 45.8%, respectively). Most reactions were reported the day after vaccine receipt and were mild.Īfter a third dose, injection-site and systemic reactions were often reported (68.5% and 45.6%, respectively).

The most common adverse reactions included injection-site pain (66.7%), fatigue (28.9%), and headache (19.9%). On May 17, the FDA authorized Pfizer COVID-19 boosters for children aged 5 to 11 who had received two doses of the same vaccine at least 5 months earlier.ĭuring the study period, v-safe received information about 3,249 third doses of the Pfizer vaccine in this age-group. VAERS is a passive vaccine surveillance system managed by the CDC and the Food and Drug Administration (FDA). In the first study, published today in Morbidity and Mortality Weekly Report, researchers from the Centers for Disease Control and Prevention (CDC) analyzed adverse-event data from the agency's voluntary smart phone-based v-safe vaccine-monitoring program and the Vaccine Adverse Event Reporting System (VAERS) from May 17 to Jul 31, 2022. Data collected from two vaccine safety surveillance programs in the first 10 weeks of administration of third doses of the Pfizer/BioNTech COVID-19 to US children aged 5 to 11 years show that serious adverse events were rare.Ī related study in South Korea shows waning vaccine effectiveness (VE) in adolescents after two and three Pfizer doses but sufficient protection against critical illness.


 0 kommentar(er)
0 kommentar(er)
